american academy of pediatrics covid vaccine under 12
Judy there are 48 million kids in America under the age of 12 but the timeline for a vaccine for those kids keeps changing. ITASCA IL -- Today the Food and Drug Administration FDA finalized the full approval of the Pfizer-BioNTech COVID-19 vaccine for ages 16 and older.

Covid Cases Among Children Jumped 84 Last Week Here Are The States Where Kid Hospitalizations Are Increasing
The American Academy of Pediatrics AAP in collaboration with the Centers for.

. Children accounted for 15 of new Covid cases reported last week American Academy of Pediatrics data analysis shows A 13-year-old shows his bandage after receiving a dose of the Covid vaccine in. On May 12 2021 CDC approved the use of the Pfizer-BioNTech COVID-19 vaccine in persons aged 12-15 years following the vaccines EUA granted by the FDA on May 10th. The reopening of society this summer and the development of viral mutations leaves those children exposed in new ways even while clinical trials are underway to develop vaccines for them.
ShopAAP is the official store of the American Academy of Pediatrics. Currently only the Pfizer COVID-19 vaccine has an emergency use authorization for children 12 and up. Children and COVID Vaccine Data Report.
Updated weekly this data report tracks the progress in vaccinating US children under 18 years of age. It is a lower dose than the 10-µg doses used for children ages 5-11 years and 30-µg doses used for those 12 years and older. In the United States recent data from the American Academy of Pediatrics show that by 21 February 2022 123 million pediatric COVID-19 cases had been reported since the onset of the pandemic which represents 157 of all confirmed cases in the country 121165.
All COVID-19 vaccines approved andor granted Emergency Use Authorization EUA by the Food and Drug Administration FDA and vaccines on the Recommended Child and Adolescent Immunization Schedule including co-administered vaccines are monitored through robust FDA and CDC systems that monitor vaccine safety in the United States. A single booster dose of the Pfizer vaccine is authorized for kids 12 through 17 years old at least 5 months after the second dose of vaccine. As COVID-19 vaccines are being administered to anyone age 12 and up Americas youngest children remain the last group that cannot get a vaccination.
The Centers for Disease Control and Prevention CDC director signed off on boosters for this group Wednesday after an advisory group voted 13-1 in favor and called for a strong recommendation. The AAP recommends COVID-19 vaccination for all children and adolescents 5 years of age and older who do not have contraindications using a COVID-19 vaccine authorized for use for their age. Is the Pfizer BioNTech mRNA vaccine.
In a New. FDA authorizes COVID-19 vaccine for adolescents ages 12-15. The AAP recommends against giving the vaccine to children under 12 until authorized by the FDA While the FDA is considering full approval of the vaccine for ages 12 through 15 it is available under emergency use authorization for this age group now and AAP strongly recommends all eligible adolescents be vaccinated as soon as possible.
Ad Maximize protection get all recommended vaccine doses and boosters as soon as possible. NPRs Ailsa Chang speaks with American Academy of Pediatrics President Lee Savio Beers about the mounting pressure to consider emergency use authorization of COVID-19 vaccines for children under 12. Neither the Johnson Johnson or Moderna vaccine have been approved for anyone under age 18.
Cases among Americas youth. The COVID shot for children 5 years to 11 years old is a lower dose than the dose recommended for people 12 years and older. AAP calls for completion of clinical trials of the vaccine in younger ages to identify correct dose.
AAP CDC recommend COVID-19 vaccine for ages 12 and older May 12 2021 In addition to approving the Pfizer-BioNTech vaccine for adolescents the CDC updated its clinical guidance to allow coadministration of vaccines. While questions may now arise about the administration of the vaccine off-label for children aged 11 and younger. Food and Drug Administration FDA to approve the use of COVID-19 vaccines in children younger than age 12.
Updated weekly find the latest data on COVID-19 cases in children by state. Were 67 000 pediatricians committed to the optimal physical mental and social health and well-being for all infants children adolescents and. The American Academy of Pediatrics AAP recommends the following related to coronavirus disease 2019 COVID-19 vaccine in children and adolescents.
The best way to slow the emergence of COVID variants is to get vaccinated. It is a lower dose than the 10-µg doses used for children ages 5-11 years and 30-µg doses used for those 12 years and older. All purchases directly benefit and support the health and well-being of all infants children adolescents and young adults.
Because theyre still growing and developing its critical that these vaccines are evaluated in well-designed and well-conducted clinical trials Children under 5 years would receive doses of 3 micrograms µg. Adolescents ages 12-15 years can begin receiving COVID-19 vaccine boosters. The American Academy of Pediatrics AAP is urging the US.
The Food and Drug Administration must work aggressively toward authorizing a Covid-19 vaccine for children under age 12 the head of the American Academy of Pediatrics wrote in a letter to Dr. The only COVID-19 vaccine presently available for children older than 5 years in the US. This vaccine safety system.
COVID Cases in Children Data Report. The viral vector vaccine in the United States is given in one dose to people 18 years and older. The American Academy of Pediatrics AAP recommends that all eligible children and adults.
The FDA issued an EUA for the Pfizer vaccine for kids aged 5 to 11 on 10292021 followed by ACIP recommendation and CDC approval on 1122021. Recent research suggests that the Pfizer-BioNTech COVID-19 vaccine authorized for emergency use in children ages 5 to 11 isnt nearly as effective as it is for adolescents or adults.

Pediatricians Plead With Fda To Move Quickly On Covid Vaccine For Kids
White House Calls On Pediatricians To Help With Rollout Of Kids Covid Vaccine

Covid And Kids Coronavirus Cases In Children Increased By Nearly 240 Since July American Academy Of Pediatrics Says Abc7 Chicago

Pediatricians Want Kids To Be Part Of Covid Vaccine Trials Kaiser Health News
Faq About Pfizer S Covid Vaccine And Kids Shots Health News Npr
American Academy Of Pediatrics Urges Fda To Approve Covid Vaccines For Children Under 12 Youtube
What Parents Should Know About Covid 19 Vaccines For Kids Under 12
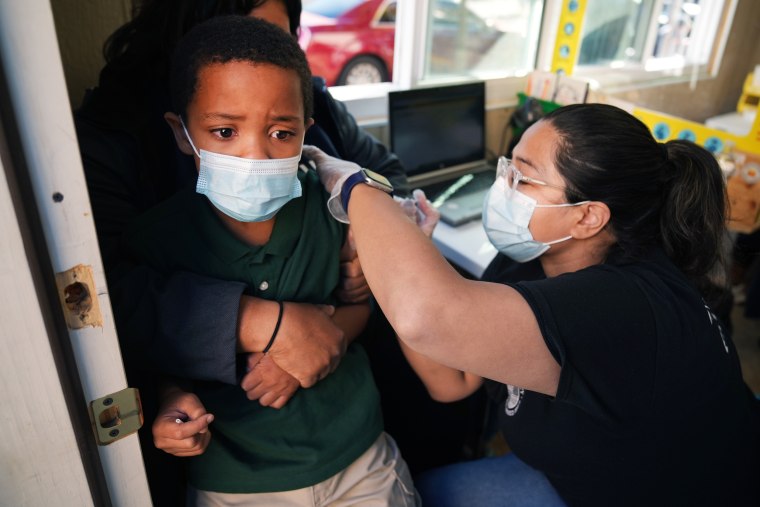
What Pediatricians Want Parents To Know About The Covid Vaccine For Kids

Massachusetts Chapter Of The American Academy Of Pediatrics Home Facebook

Faq Covid 19 Vaccine For Kids Under 12 Lurie Children S

Are Kids At Higher Risk For Re Infection Of Covid 19

Back To School White House Pushes For Kids 12 And Up To Get Covid Vaccine

American Academy Of Pediatrics What Do Parents Need To Know About The Covid Vaccine For Kids It S Safe Kids Ages 5 11 Get A Smaller Dose It Protects Against Long Term Side Effects

What To Know About Children 5 11 Getting The Covid Vaccine The Washington Post

Aap Recommends Covid 19 Vaccine For Ages 16 And Up
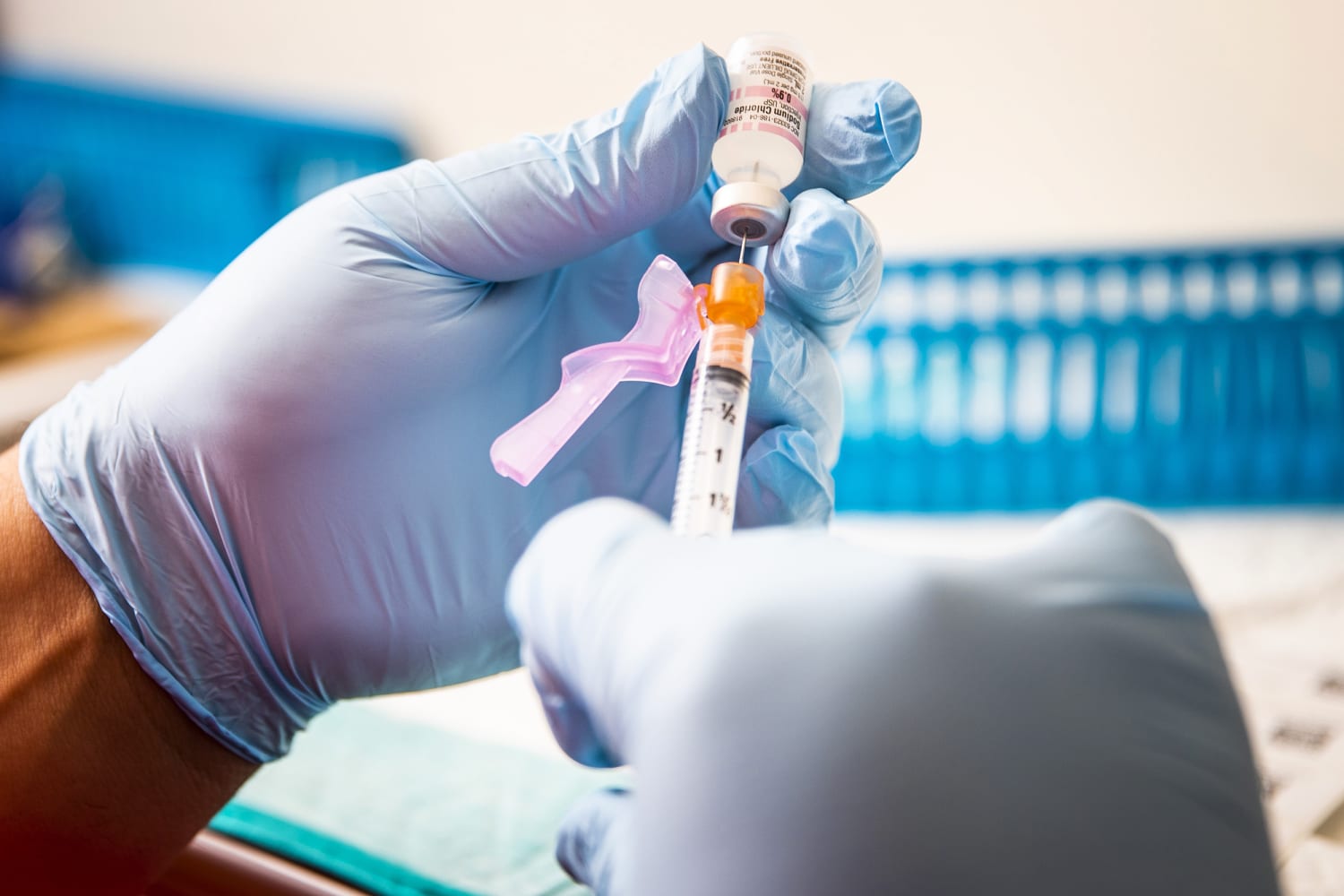
Covid Vaccines For Kids Under 12 Expected Midwinter Fda Official Says
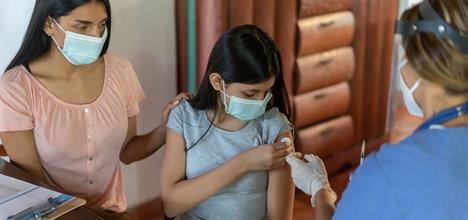
Aap Advises Children And Teens Age 12 And Up Get The Covid 19 Vaccine Healthychildren Org

More Children Are Catching Covid 19 But It S Unclear If They Re Getting Sicker Coronavirus Updates Npr

Pediatricians Besieged By Parents Seeking Coronavirus Shots For Kids Under 12 The Washington Post